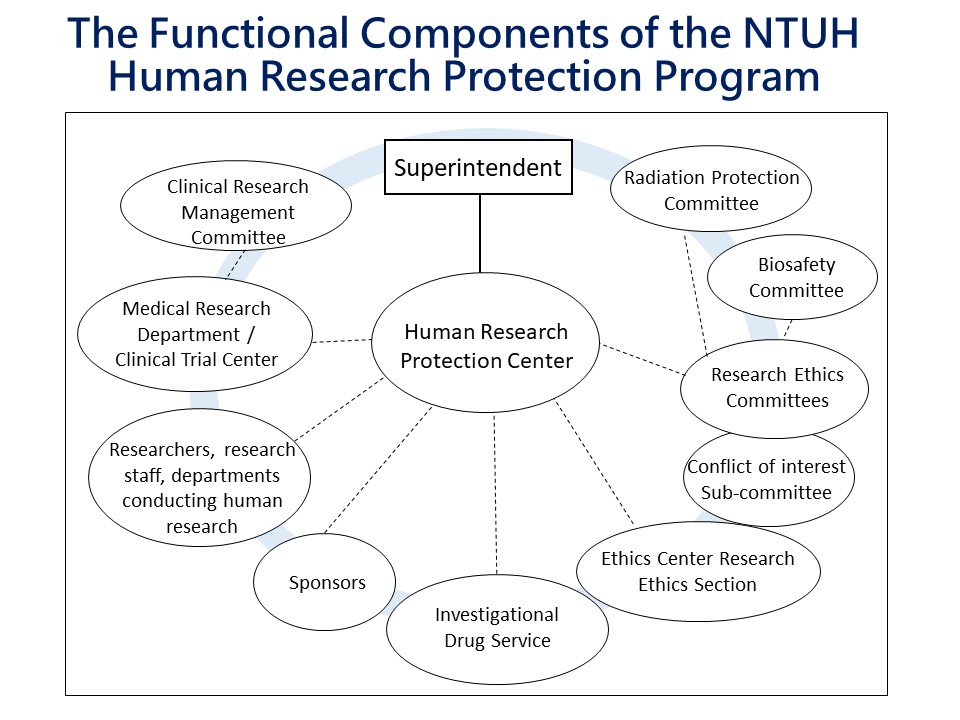
NTUH has set up a robust human research protection program combining existing research subject protection functions, including review of protocols, HRPP related training & education, audit & quality improvement and contract review. These functions are performed by various departments, such as Clinical Trial Centers and Research Ethics Committees. The Human Research Protection Center has been added to the program to oversee human subject protection affairs and coordinate among component units where necessary.